 Needles and syringes need to perform precisely to specification or they may seriously limit the ability of a health care professional to effectively and safely treat their patient. Force and torque testing in development and production enables the manufacturers to guarantee consistent quality and performance of their products. This article examines seven typical testing applications within this field.
Needles and syringes need to perform precisely to specification or they may seriously limit the ability of a health care professional to effectively and safely treat their patient. Force and torque testing in development and production enables the manufacturers to guarantee consistent quality and performance of their products. This article examines seven typical testing applications within this field.
By David Mercer
Billions of syringes and single-use needles are produced annually, from hypodermic and surgical needles, to lancets and winged infusion sets. With ever-increasing demand to drive down mass production costs, and the frequent introduction of new safety-oriented devices, it is a continuously developing market. Assessing the mechanical properties of needles and syringes is a crucial ingredient in the successful development and production of such devices. Force and torque testing is a simple, fast and relatively inexpensive method of quantifying the critical physical attributes of these products. It allows manufacturers to quickly identify and amend production errors that not only harm the brand’s reputation, but also jeopardize the safety and comfort of the patient. It also helps designers to perfect the usability and fitness-for-purpose of their devices while striking an optimum balance between material usage and mechanical strength, and ensuring all new products conform to international standards. The following examines seven key application areas for force and torque testing of needles and syringes, considering, in turn, the purpose, benefits, and considerations of each test, and where appropriate, describing, how they are typically performed.
The peel strength of an adhesive-sealed syringe packaging is commonly tested to ensure the device is secure and sterile, while remaining easily accessible on demand.
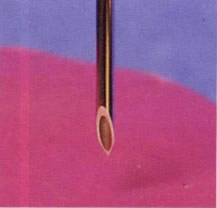
To maximize patient comfort, it is essential that a clean insertion and extraction of the needles is achieved, resulting in minimum trauma to the epidermis and subcutaneous tissue. Malformed and insufficiently sharp needles points can cause considerable pain on insertion, easily preventable with a well-designed and consistently produced device. Needle penetration and extraction forces are measured in development and production to characterize point sharpness, the frictional forces experienced along the shaft of the needles, and the effectiveness of the bevelled tip geometry. The test typically requires a motorized force testing system, with a specialized lower fixture to hold in place a stretched foil of PBC or PU over a steel ring to simulate skin. The needle, which is mounted onto a sensitive, low capacity load cell, is brought down through the foil at a constant rate by the system’s motorized crosshead travel. A computer-controlled system will provide a real-time graphical force profile, which may be analyzed for key areas of interest, including:
1) The initial peak force at which the needle tip first penetrates the foil.
2) The force required for the bevelled edge to continue through the hole, cutting it wider.
3) The frictional force experienced as the needle shaft slides through the hole, once the bevel has been passed.
Needles are held within their hub by a variety of methods, including bonding, moulding, welding, mechanical interlocking, and sealing. The retention strength of the assembly method must be sufficiently high to avoid disassembly or seal failure in use. To test retention strength, the test system is normally fitted with an aluminium mounting block, beneath which the hub is placed with the needle tip exposed vertically. In the case of smaller needles and lancets, a lever-operated pin vice may be fitted to the tip, and an axial tensile load applied at a constant rate, until the needle is completely dislodged from the hub. For larger gage needles, a small wedge grip may be required to hold the sample. This test is performed in conformance with international standard ISO 7864.
Single-use surgical needles are supplied connected to a length of suture via a crimped end. This may take the form of a drilled-end crimp, In which a small aperture is made in the end of the needle, the suture threaded inside, and the needle passed around it, Alternatively, it may be a flange-type crimp, in which the needle end is pressed into a semi-circular formation, the suture placed into the groove, and either end of the semi-circle closed around it. The pull-off force of the needle from the suture must be sufficiently high to ensure it does not come away during placement of the sutures, but sufficiently low to enable surgeons to pull the needle from the suture as required. To perform the pull-off test, the needle is held in a small wedge grip, while the suture material is wrapped around a cable cam grip. This spreads the load evenly to avoid slippage during testing. A tensile load is then applied at a slow, steady rate until the suture is dislodged from the crimp. The maximum tensile force experienced is recorded as the crimp retention strength.
The steel needle itself must be produced to a high standard of quality. Force testing should play an integral role in both productions (as inline checks at steel formers and supplied materials quality checks at assemblers), and in development, to identify opportunities to reduce material usage without compromising functionality.
This mechanical test enables quantification of the material properties of the needle, and identification of areas of recurring weakness. The steel needle is held at either end by pneumatic grips, which exert an adjustable pressure suited to the gage of needle, eliminating early failure at the jaw face, It is common to thread small inserts into the needle ends to avoid collapse. The pneumatic grips should have lightly serrated faces to avoid sample slippage under loading. An axial tensile load is steadily applied until a clean break occurs in a central portion of the needle, clear of the grips.
The three-point-bend test is used to assess the stiffness of the needle, and hence the likelihood of permanent deformation on insertion. The test requires the use of a specialized fixture, comprising two adjustable lower support anvils on which the sample is placed, and a single upper anvil mounted onto the motorized crosshead of an automated testing machine. The upper anvil descends to a pre-determined height, effectuating a bend in the centre of the sample. Analysis of the load at a carrying vertical displacement allows calculation of the Young’s Modulus of the needle, a useful measure for characterizing its expected behaviour under typical loading in use.
 |
 |
| Penetration testing of hypodermic needles assesses point sharpness and geometry. | Needle hub pull-out tests commonly assess joint strength in development and production |
 |
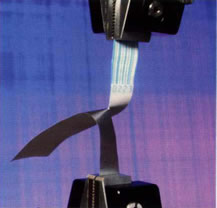 |
| Threaded Luer lock connectors undergo torque testing to assess joint strength and ease of operation. | The tear strength of device packaging material is a critical parameter in assuring quality in production. |
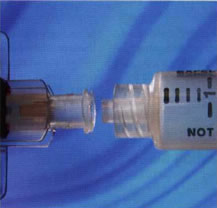
Needle hubs attach to syringes (and other devices) via push/pull taper Luer, or threaded Luer lock connectors. The force or torque required to disassemble the two elements must be high enough to avoid failure in operation and ensure a hermetic seal in the connection, yet low enough to enable quick and easy engagement and disassembly as required by the healthcare professional. In the case of Luer locks, torque assessment should be performed on a low-level torque testing system, with a motorized base plate and a low capacity (e.g. 1.5 Nm) torque transducer. Universal ripping pegs may be used to hold the sample in place, although customized fixtures ensure greater accuracy and repeatability. the most common test involves tightening the Luer lock connection between a needle and a fluid-filled syringe to a pre-determined torque, and visually inspecting the joint for leakage. This test is described in international standard ISO 594 1/2.
Syringes must deliver and extract fluids in a smooth, controlled manner. A syringe plunger that is too easy or hard to actuate, or that stalls and judders on depression will not perform reliably during injection or aspiration. The efforts required to expel liquid from the syringe, and draw liquid into the syringe, are known as the expression force and aspiration force respectively. These will be determined by a range of variables, including the viscosity of the liquid, the size of the syringe/needle aperture, the ‘fit’ of the plunger within the syringe barrel and the density of the tissue substrate. The syringe must be developed with the optimum balance of these variables to assure consitency and usability. International standard ISO 7886 describes a method for determining aspiration and expression forces using a syringe half-filled with water, vertically mounted onto a motorized force testing system. The syringe outlet is connected to a reservoir (open to atmospheric pressure), with the water level aligned to fill level in the syringe chamber. The piston is held from below by a suitable lower fixture. On initiation, the system pulls the syringe plunger down at a constant rate, drawing fluid in the chamber, and records the aspiration force. The motor is then reversed to record the expression force. Anomalous peaks and troughs in the force profile are monitored to ensure the syringe provides a smooth motion of aspiration and expression. Other common force tests performed on syringes include chamber stress cracking to assess compression resistance and disassembly force testing of the rubber plunger from the piston.
Penetration testing of hypodermic needles assesses point sharpness and geometry.
Threaded Luer lock connectors undergo torque testing to assess joint strength and ease of operation.
Needle and syringe packaging must ensure the device remains secure, sterile, and undamaged during transportation and storage, while remaining easily accessible on demand. Force and torque testing in design, production, and processing greatly aids the creation of user-friendly and consistently reliable packaging. Typical applications include:
1) Tensile strength, elongation, and tear testing of flexible packaging material (in line with ISO 11607 and EN 869-1 standards).
2) Peel strength of adhesive sealed needle and syringe packs to qualify ‘openability’.
3) Pierce testing of films and foils used on needle blister packs.
4) Co-efficient of friction assessment of packaging material to optimize form-fill-seal machinery settings.
There are many useful force and torque testing applications throughout the lifecycle of needle and syringe devices; from forming the needle, joining it to its hub, and packaging it in the factory to unwrapping the needle, attaching it to a syringe, and injecting a patient at the hospital. Developers and producers alike should take advantage of these tests as a fast, inexpensive, yet accurate method of assessing the quality, usability, and conformance of their products to international standards.